Phase Changes
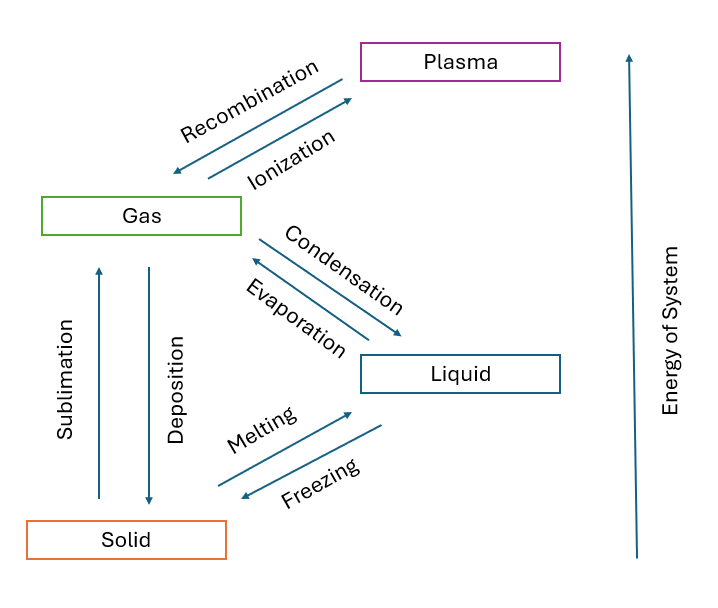
As the energy within a substance becomes greater and the temperature rises, the particles within the substance begin to move more freely, and vice versa. This changes the structure and properties, or phase, of the substance. While most substances undergo phase changes in a linear fashion, the liquid phase is sometimes skipped due to a lack of atmospheric pressure. The diagram at right maps how substances change phases.
